Urothelial Carcinoma Drug Pipeline Analysis Overview
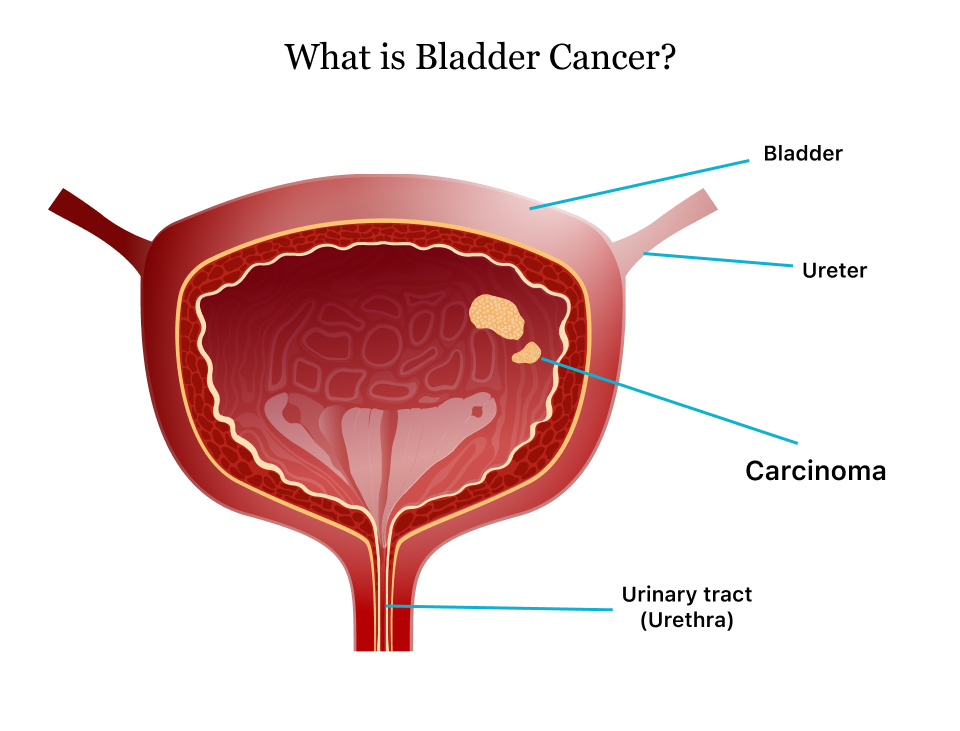
Urothelial carcinoma (UC), also referred to as transitional cell carcinoma, arises from the cells lining the bladder, ureters, or kidneys. The disease often presents with haematuria, urgency, and dysuria, but can be asymptomatic in its early stages, making early detection challenging. Urothelial Carcinoma Despite advances in cancer research, metastatic UC remains a high unmet medical need, as current treatment options are limited, particularly for patients with advanced or recurrent disease.
Get a Free Sample Report with a Table of Contents: https://www.expertmarketresearch.com/clinical-trials/urothelial-carcinoma-drug-pipeline-analysis/requestsample
The drug pipeline for urothelial carcinoma is rich with innovative therapeutic approaches, with several novel treatments undergoing clinical trials to address this urgent need. These therapies include targeted therapies, immunotherapies, and novel chemotherapies designed to improve overall survival, reduce relapse rates, and mitigate treatment-related side effects.
Urothelial Carcinoma Drug Pipeline Analysis Dynamics
The dynamics of the urothelial carcinoma drug pipeline are shaped by several critical factors:
- Unmet Need: As mentioned, UC is often diagnosed at an advanced stage, leaving few treatment options for patients once the disease becomes metastatic. Despite the approval of immune checkpoint inhibitors, such as pembrolizumab (Keytruda) and atezolizumab (Tecentriq), the clinical benefit remains modest, driving the need for newer therapies that target the underlying mechanisms of the disease.
- Emerging Treatments: The development of immunotherapies, including antibody-drug conjugates (ADCs) and gene therapies, is at the forefront of UC drug development. Additionally, combination therapies combining immune checkpoint inhibitors with chemotherapy or targeted therapies are gaining significant attention.
- Regulatory Environment: The fast-paced regulatory approvals for immunotherapy drugs by global health authorities, including the FDA and EMA, have been encouraging. This trend supports the accelerated development of novel UC treatments that address unmet medical needs, especially in the metastatic setting.
- Clinical Trials: Clinical trials play a pivotal role in advancing UC drug discovery. Key phases of these trials focus on assessing the efficacy, safety, and optimal dosing regimens of novel agents. The success of ongoing phase II and III trials could significantly change the therapeutic landscape.
Read Full Report with Table of Contents: https://www.expertmarketresearch.com/clinical-trials/urothelial-carcinoma-drug-pipeline-analysis
External Urothelial Carcinoma Drug Pipeline Analysis Trends
External trends influencing the development of drugs for urothelial carcinoma include:
- Rise in Cancer Incidence: The increasing global incidence of UC, particularly in older populations, drives the demand for new treatments. Ageing populations in high-income countries are particularly affected, with lifestyle factors such as smoking playing a significant role in its rise.
- Technological Advancements: Advances in biomarker discovery, precision medicine, and genomics have opened new frontiers in UC treatment. Targeted therapies based on genetic mutations and molecular profiling are expected to improve clinical outcomes and reduce toxicity.
- Partnerships and Collaborations: Pharmaceutical companies, including major players like Bristol-Myers Squibb, Bayer, and Hoffmann-La Roche, are increasingly entering into partnerships with biotech firms to expedite the development of novel UC therapies. These collaborations enhance research capabilities and increase the speed at which promising candidates move through clinical trials.
- Patient-Centric Approaches: The shift towards more personalized treatment regimens, which consider individual patient profiles and genetic makeup, has influenced the development of new therapies tailored to specific subtypes of UC.
Urothelial Carcinoma Drug Pipeline Analysis Segmentation
The UC drug pipeline can be segmented based on various factors, including the type of treatment, stage of the disease, and molecular targets:
- By Treatment Type:
- Chemotherapy: Conventional chemotherapy remains a cornerstone of UC treatment, especially for advanced cases. Platinum-based chemotherapy drugs like cisplatin and carboplatin are widely used, though they are often associated with significant side effects.
- Immunotherapy: Immune checkpoint inhibitors, such as PD-1/PD-L1 inhibitors (pembrolizumab, nivolumab), have revolutionized treatment, offering prolonged survival in patients with metastatic disease.
- Targeted Therapy: Targeting specific genetic mutations or cell signaling pathways is a promising approach. For instance, fibroblast growth factor receptor (FGFR) inhibitors are being investigated in clinical trials.
- Combination Therapy: The combination of immunotherapy with chemotherapy or targeted therapies aims to increase efficacy and overcome resistance mechanisms.
- Chemotherapy: Conventional chemotherapy remains a cornerstone of UC treatment, especially for advanced cases. Platinum-based chemotherapy drugs like cisplatin and carboplatin are widely used, though they are often associated with significant side effects.
- By Disease Stage:
- Non-Muscle Invasive UC (NMIBC): This early-stage disease is often treated with intravesical therapy, including Bacillus Calmette-Guerin (BCG) therapy and novel immunotherapies.
- Muscle-Invasive UC (MIBC): For patients with MIBC, surgical intervention (cystectomy) is typically followed by adjuvant chemotherapy or immunotherapy.
- Metastatic UC: The most challenging form of the disease, metastatic UC is treated with systemic chemotherapy, immunotherapy, and experimental therapies aimed at improving overall survival.
- Non-Muscle Invasive UC (NMIBC): This early-stage disease is often treated with intravesical therapy, including Bacillus Calmette-Guerin (BCG) therapy and novel immunotherapies.
- By Molecular Target:
- FGFR Inhibitors: Targeting the FGFR pathway is a promising therapeutic approach, with drugs such as erdafitinib in clinical trials.
- HER2 Inhibitors: HER2-targeted therapies, similar to those used in breast cancer, are under investigation for UC.
- PD-1/PD-L1 Inhibitors: These inhibitors are at the forefront of immunotherapy for UC, enhancing the body’s immune response against tumour cells.
- FGFR Inhibitors: Targeting the FGFR pathway is a promising therapeutic approach, with drugs such as erdafitinib in clinical trials.
Urothelial Carcinoma Drug Pipeline Analysis Growth
The urothelial carcinoma drug pipeline is expected to experience significant growth in the coming years. A few key factors driving this growth include:
- Expanding Research: Continued investment in UC research, particularly in the immuno-oncology space, will lead to the discovery of more effective treatments, improving survival rates and quality of life for patients.
- Technological Innovation: Advances in artificial intelligence (AI) and machine learning are accelerating the identification of new drug candidates and optimizing clinical trial designs.
- Increased Market Competition: As more pharmaceutical companies enter the UC space, competition is expected to drive innovation and enhance the availability of more treatment options.
- Government Support and Funding: Public and private sectors are increasing funding for cancer research, which could lead to quicker advancements in the treatment of UC.
Recent Urothelial Carcinoma Drug Pipeline Analysis Market Developments
Recent developments in the urothelial carcinoma drug pipeline include the approval of several key immunotherapies, such as Keytruda and Tecentriq. These approvals have helped extend survival for patients with metastatic UC. Furthermore, emerging therapies like erdafitinib, a targeted FGFR inhibitor, have shown promising results in clinical trials, offering hope for patients with resistant disease.
Additionally, ongoing clinical trials exploring the efficacy of combination therapies, such as nivolumab combined with ipilimumab, are generating excitement for their potential to enhance clinical outcomes.
Urothelial Carcinoma Drug Pipeline Analysis Scope
The scope of the urothelial carcinoma drug pipeline is broad, encompassing a wide range of therapies designed to improve patient outcomes. The key areas of focus include:
- Immunotherapy Advancements: The development of immune checkpoint inhibitors and novel immune therapies is likely to lead the way in improving survival rates.
- Targeted Therapy: Therapies targeting specific genetic mutations or molecular pathways are gaining traction and could revolutionize UC treatment.
- Combination Therapies: The combination of chemotherapy, immunotherapy, and targeted treatments is expected to become a cornerstone of future UC treatment regimens.
- Personalized Medicine: Personalized and precision medicine approaches, tailored to individual patient profiles, are set to play an increasingly important role in UC treatment.
Urothelial Carcinoma Drug Pipeline Analysis and COVID-19 Impact
The COVID-19 pandemic significantly impacted the clinical development of new therapies for urothelial carcinoma. Delays in clinical trials, interruptions in patient recruitment, and shifts in healthcare priorities delayed the progression of several therapies. However, the pandemic also highlighted the need for novel, more accessible treatments, particularly those that can be administered remotely or as part of outpatient care.
Key Players in the Urothelial Carcinoma Drug Pipeline
Several key players are actively involved in the development of new therapies for urothelial carcinoma, including:
- Bristol-Myers Squibb: A major player in the immuno-oncology space, Bristol-Myers Squibb is developing several PD-1/PD-L1 inhibitors, including nivolumab, which has shown promise in clinical trials for UC.
- Bayer: Bayer is exploring a range of therapies for UC, including targeted therapies that address genetic mutations driving cancer progression.
- Hoffmann-La Roche: Roche’s pipeline includes innovative cancer immunotherapies and targeted treatments designed to treat urothelial carcinoma, with a focus on expanding options for metastatic disease.
FAQ
1. What is urothelial carcinoma?
Urothelial carcinoma is the most common type of bladder cancer, affecting the cells lining the bladder, ureters, and kidneys. It is characterised by poor